Where Is All the Xenon?
Neon (Ne), Krypton (Kr) and Xenon (Xe) are referred to as the rare gases, as they comprise a minimal amount of the earth’s atmosphere. These rare gases are produced in the same cryogenic separation and purification process that produces Oxygen, Nitrogen and Argon but in much smaller quantities. Of these rare gases, Xenon is the rarest, found in the earth’s atmosphere at only 1 part per 20 million. However, scientists analyzed early meteorite material and they discovered that Xenon levels in the current atmosphere are more than 90 percent less than they would have predicted. So, what happened to all the Xenon?

History of Xenon
The name derives from the Greek xenos, which means “strange” or “foreign”. In the late 1800s, two chemists in the UK, Morris Travers and William Ramsay, discovered that Argon, Neon and Krypton could be extracted from liquid air. During further experimentation in 1898, the pair noticed a curious blue glow from a heavier gas while distilling Krypton using a liquid-air machine. It was then they were introduced to Xenon. They also realized it was yet another member of the inert group of “gaseous elements”, as they were then known, because of their lack of chemical reactivity. The earth’s atmosphere is about 0.0000087% Xenon compared to 0.001818% Neon and 0.000114% Krypton.

Current Xenon Uses
More than 4.5 times heavier than air, Xenon is colorless, odorless, and tasteless. Xenon is unique in that it is used for launching and landing space shuttles. It also propels ion thrusters and due to its inert nature, it presents no risk of explosion when ions are discharged. Its blue hue can be sighted on spacecraft or airport runways, and it brings illumination to space odysseys and the abyss of the ocean for deep sea exploration. As with Krypton, it is used in lighting of many kinds, including car headlights, arc-lamps for motion pictures, and in tanning beds. Its effects as a general anesthetic agent are well known and Xenon is now being used in medical imaging.
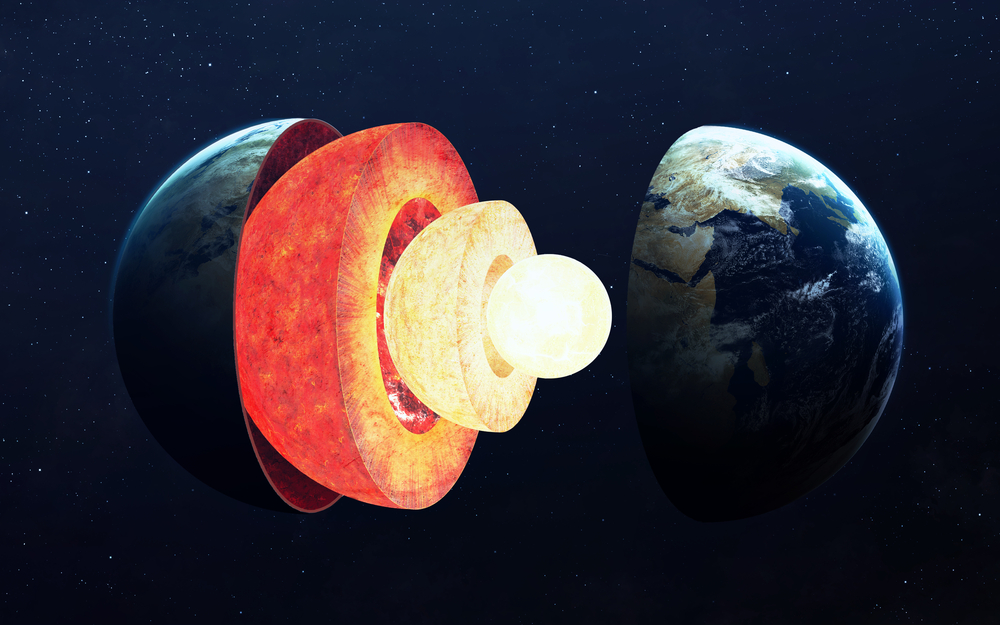
Where is the Xenon?
Scientists typically fall into two camps regarding the “missing” Xenon. The first believe that Xenon escaped from the Earth’s atmosphere into space during a process called degassing, as it’s also found on Mars and other Martian territories in larger amounts. However, others think this theory is highly unlikely, as Xenon is quite heavy and many of its lighter counterparts are still abundantly available. Perhaps the second group has the most likely hypothesis? Their belief is that Xenon is being held in the Earth’s core which contains about one-third of the planet’s mass and is made of iron and nickel. Most noble gases do not bond with other materials, but in comparison, Xenon has a large atomic radius and does form bonds with other elements. However, some have debated that it would not bond with the elements iron and nickel, suggesting this is also unlikely.
The Research Continues
Convinced that the missing Xenon is in the Earth, Yanming Ma, a computational physicist and chemist at Jilin University in Changchun, China leads a team of researchers in studies about this theory. Their calculations suggest that at the extreme temperatures and pressures found in Earth’s core, Xenon can bond with both iron and nickel to form a compound. “We do hope future high-pressure experiments can be carried out to confirm our predictions,” Ma said.
However, the experiments won’t be easy. Extremely high temperatures of more than 6,000 Kelvin (10,340 degrees Fahrenheit) must be applied and controlled for a successful outcome. Does Earth’s core hold all the “missing” Xenon? Perhaps these experiments and further exploration will provide us with our answer.
Rare Gas Supplier in the Rocky Mountain Region
Though these gases are rare, Rocky Mountain Air supplies Neon, Krypton, and Xenon to the Rocky Mountain region (Colorado, Utah, Wyoming, Idaho, Nebraska) and we offer both high-purity cylinders and mixtures to serve a multitude of industries, from lighting to aerospace, to medicine. If you are interested in partnering with RMA as your rare gas supplier, please contact your local branch to speak to a representative today. We look forward to serving you with flawless dependability!